VIDAS® B•R•A•H•M•S PCT™
PCT-Guided Antibiotic Therapy
Procalcitonin (PCT) is a biomarker that can help:
- Assess initial risk of sepsis
- Monitor patient progress and mortality risk during treatment for sepsis
- Determine whether to start antibiotics for
Lower Respiratory Tract Infection (LRTI) - Determine when to discontinue antibiotics
Results within 20 minutes.
For More
Information
VIDAS B·R·A·H·M·S PCT is an automated test for use on the instruments of the VIDAS family of instruments for the determination of procalcitonin in human serum or plasma (lithium heparinate) using the ELFA (Enzyme-Linked Fluorescent Assay) technique.
Used in conjunction with other laboratory findings and clinical assessments, VIDAS B•R•A•H•M•S PCT is intended for use as follows:
- to aid in the risk assessment of critically ill patients on their first day of ICU admission for progression to severe sepsis and septic shock
- to aid in assessing the cumulative 28-day risk of all-cause mortality for patients diagnosed with severe sepsis or septic shock in the ICU or when obtained in the emergency department or other medical wards prior to ICU admission, using a change in PCT level over time
- to aid in decision making on antibiotic therapy for patients with suspected or confirmed lower respiratory tract infections (LRTI) - defined as community-acquired pneumonia (CAP), acute bronchitis, and acute exacerbation of chronic obstructive pulmonary disease (AECOPD) - in an inpatient setting or an emergency department
- to aid in decision making on antibiotic discontinuation for patients with suspected or confirmed sepsis
VIDAS B·R·A·H·M·S PCT is not indicated to be used as a stand-alone diagnostic assay. PCT results should always be interpreted in the context of the clinical status of the patient and other laboratory results. PCT levels for the prediction of mortality and overall mortality are strongly dependent on many factors, including pre-existing patient risk factors and clinical course.
VIDAS B·R·A·H·M·S PCT is available to order now via our eCommerce store.
Disclaimer: All VIDAS® instruments and assays are to be run by laboratory personnel in a laboratory certified under CLIA or by a CLIA approved State laboratory program. All personnel tasked with operating the VIDAS® systems should be qualified and undergo a yearly competency assessment as defined in 42 CFR 493 subpart M “Personnel for Nonwaived Tests."
The VIDAS B•R•A•H•M•S PCT assay is compatible with the following VIDAS instruments:
Storage Conditions
- Store the VIDAS B·R·A·H·M·S PCT (PCT) kit at 2-8°C.
- Do not freeze reagents, with the exception of calibrators and controls after reconstitution.
- Store all unused reagents at 2-8°C.
- After opening the kit, check that the SPR® pouch is correctly sealed and undamaged. If not, do not use the SPRs.
- Carefully reseal the pouch with the desiccant inside after use to maintain stability of the SPRs and return the complete kit to 2-8°C.
- If stored according to the recommended conditions, all components are stable until the expiration date indicated on the label. Refer to the kit composition table for special storage conditions.
Disclaimer: All VIDAS® instruments and assays are to be run by laboratory personnel in a laboratory certified under CLIA or by a CLIA approved State laboratory program. All personnel tasked with operating the VIDAS® systems should be qualified and undergo a yearly competency assessment as defined in 42 CFR 493 subpart M “Personnel for Nonwaived Tests."
PCT-Guided Antibiotic Therapy for Sepsis
For more information, please see full package insert for VIDAS B•R•A•H•M•S PCT (30450-01).
VIDAS B•R•A•H•M•S PCT can help clinicians make better-informed decisions and help improve accuracy of early sepsis diagnosis, using procalcitonin to provide critical biomarker information.
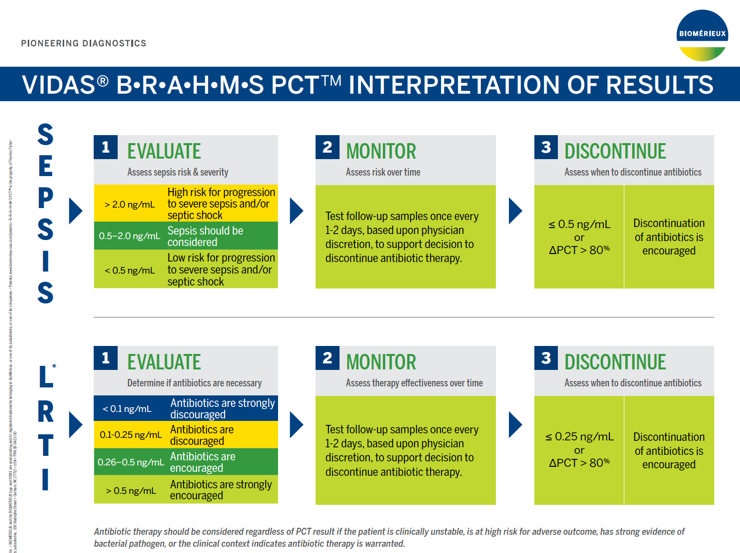
1. Evaluate: Assess Sepsis Risk & Severity
In just 20 minutes, you can get results from a proven, sensitive biomarker that provides specific information that adds to physician acumen, and enables more appropriate triage decisions.
| >2.0 ng/mL | High risk for progression to severe sepsis and/or septic shock |
| 0.5 - 2.0 ng/mL | Sepsis should be considered |
| <0.5 ng/mL | Low risk for progression to severe sepsis and/or septic shock |
2. Monitor: Assess Risk Over Time
Assessing PCT kinetics over time provides valuable information regarding patient disposition, response to treatment and likelihood of survival.
Follow up samples should be tested once every 1-2 days, based upon physician discretion taking into account patient's evolution and progress.
3. Discontinue: Assess When to Discontinue Antibiotics
VIDAS B•R•A•H•M•S PCT has been cleared by the FDA to aid in decision making on antibiotic discontinuation for patients with suspected or confirmed sepsis.
Antibiotic therapy may be discontinued if the PCT concentration is ≤0.50 ng/mL or if the ∆PCT >80%.
Antibiotic therapy may be continued based upon other clinical findings, such as failure to control a local infection, or ongoing physiological instability.
PCT-Guided Antibiotic Therapy for Lower Respiratory Tract Infection
50% of antibiotics prescribed for acute respiratory conditions are unnecessary.1 LRTI can present in many different ways: acute bronchitis, community acquired pneumonia, and acute exacerbation of COPD. Clinical characteristics are non-specific: cough, sputum, fever, and shortness of breath. In the past, this ambiguity was met with almost-automatic antibiotic therapy. Today we have better information and better ways to decide if antibiotics are warranted.
Procalcitonin (PCT) provides critical biomarker information. PCT is produced by numerous organs at a cellular level after bacterial pro-inflammatory stimulation.2,3
- PCT rises in 3 - 6 hours
- Half-life of 20 - 24 hours
- Early identification & risk assessment
1. Start or Not: Know With Confidence
VIDAS B•R•A•H•M•S PCT has been cleared by the FDA to aid in decision-making on antibiotic therapy — specifically for inpatient or emergency department settings in patients with suspected or confirmed lower respiratory tract infections (LRTI), defined as community-acquired pneumonia (CAP), acute bronchitis, and acute exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD).
| PCT Value |
Reccomendation on the Initiation of Antibiotic Therapy |
| <0.10 ng/mL | Antibiotic therapy strongly discouraged. |
| 0.10 - 0.25 ng/mL | Antibiotic therapy discouraged. |
| 0.26 - 0.50 ng/mL | Antibiotic therapy encouraged. |
| >0.50 ng/mL | Antibiotic therapy strongly encouraged. |
2. When to Stop: Gain Valuable Information
PCT kinetics over time in conjunction with clinical assessments provides valuable information regarding response to treatment and can support decision-making on antibiotic discontinuation for patients with LRTI.
Antibiotic therapy may be discontinued if the PCT concentration is ≤0.50 ng/mL or if the ∆PCT >80%.
Antibiotic therapy may be continued based upon other clinical findings, such as apparent progression on chest x-ray or ongoing/increasing toxicity.
This new indication is important because antibiotic overuse is a serious problem. For each patient, improper use of antibiotics exposes patients to the risk of antibiotic-associated infections such as Clostridium difficile, and other adverse effects.4,5 For the healthcare system, there is an overall safety risk due to the rise of antibiotic resistance, with 2 million illnesses and roughly 23,000 deaths per year in the U.S.6
References:
5. Fernanda C. Less, M.D., M.P.H., Yi Mu, Ph.D., Wendy M. Bambery, M.D., et al. Burden of Clostriduim difficile Infection in the United States. N Engl J Med. 2015; 372:825-834.
Disclaimer: All VIDAS® instruments and assays are to be run by laboratory personnel in a laboratory certified under CLIA or by a CLIA approved State laboratory program. All personnel tasked with operating the VIDAS® systems should be qualified and undergo a yearly competency assessment as defined in 42 CFR 493 subpart M “Personnel for Nonwaived Tests."
What if you had an extra piece of information to help assess sepsis patients?
PROCALCITONIN: the “extra piece” that can make the difference
Procalcitonin (PCT) is often increased during systemic bacterial infection and sepsis. This biomarker is recognized as a useful tool in the diagnostic process.
VIDAS B·R·A·H·M·S PCT is an innovative procalcitonin assay. This assay is performed on the VIDAS® system, which is fully adapted for emergency or stat conditions where rapid turnaround time is important.
What is PCT?
PCT is the prohormone of calcitonin (CT). Whereas CT is secreted by the C-cells of the thyroid after hormonal stimulation, PCT can be produced by numerous cell types and organs after proinflammatory stimulation, especially when caused by bacterial challenge.1
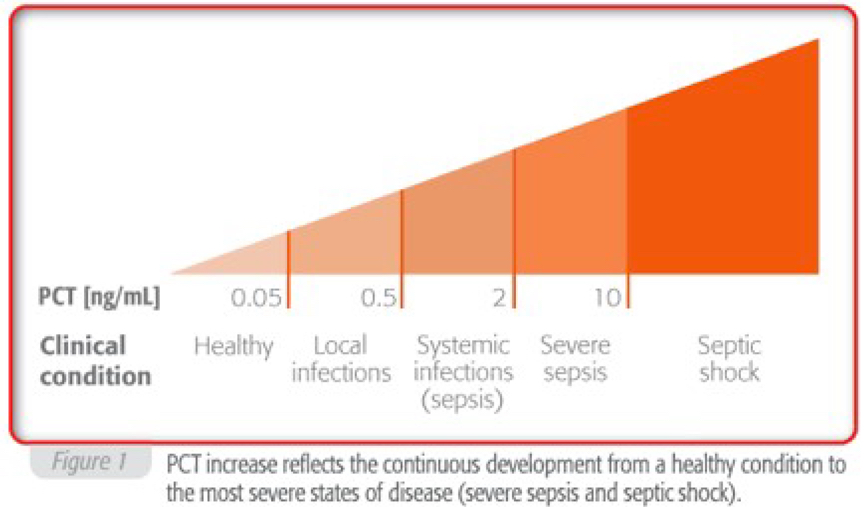
In healthy people, plasma PCT concentrations are found to be below 0.05 ng/ml, but can increase up to 1,000 ng/ml in patients with severe sepsis or septic shock.
Elevated PCT levels indicate bacterial infection accompanies by a systemic inflammatory reaction.
Localized infections do not generally cause circulating PCT increases. Slightly elevated PCT concentrations are observed in bacterial infections with minor systemic inflammatory response.
Very high values have been observed during acute disease conditions with severe systemic reactions to infection, in cases of severe sepsis or septic shock.
1. Christ-Crain M, Müller B. Procalcitonin in bacterial infections – hype, hope or more or less? Swiss Med Wkly 2005; 135: 451-60.
Disclaimer: All VIDAS® instruments and assays are to be run by laboratory personnel in a laboratory certified under CLIA or by a CLIA approved State laboratory program. All personnel tasked with operating the VIDAS® systems should be qualified and undergo a yearly competency assessment as defined in 42 CFR 493 subpart M “Personnel for Nonwaived Tests."
PCT-Guided Antibiotic Therapy for Patients with Sepsis
PCT-Guided Antibiotic Therapy for Patients with Lower Respiratory Tract Infection
Procalcitonin: Taking Antimicrobial Stewardship Up a Notch
PCT for Assessing Patient Prognosis, Response to Therapy and Guiding Antibiotic Duration in the ICU
Meisner M. Procalcitonin: Biochemistry and Clinical Diagnosis. UNI-MED Verlag AG. 2010.
Disclaimer: The inclusion of a website link or resource material for download not original to bioMérieux Inc. is not intended as an endorsement of any product, service or advertisement and bioMérieux does not guarantee the accuracy of content. When users leave the bioMérieux website through those links, they are subject to the privacy and security policies of the owners/sponsors of the outside website. bioMérieux is not responsible for transmissions users receive from linked websites.