VITEK® MS Reagents/Accessories
Ready-to-use reagents for microbial identification
Ready-to-use, light-stable reagents and accessories for safe, easy use with VITEK MS microbial identification instrument.
- Reagent kits contain all reagents needed for ID testing
- No extra reagent preparation time required
- Target slides are barcoded for complete traceability of samples
- Safe disposal and no time-consuming cleaning
Overview
VITEK MS is an automated microbial identification system that provides identification results in minutes using an innovative mass spectrometry technology — Matrix Assisted Laser Desorption Ionization Time-of-Flight, or MALDI-TOF.
Increase productivity, safety and performance using reagents and accessories designed for microbial identification with the VITEK MS MALDI-TOF Mass Spectrometry System.
- Standardized reagents eliminate the need for in-house reagent preparation and QC studies
- Barcoding for complete traceability
- Safe disposal without the need for time-consuming cleaning using hazardous chemicals
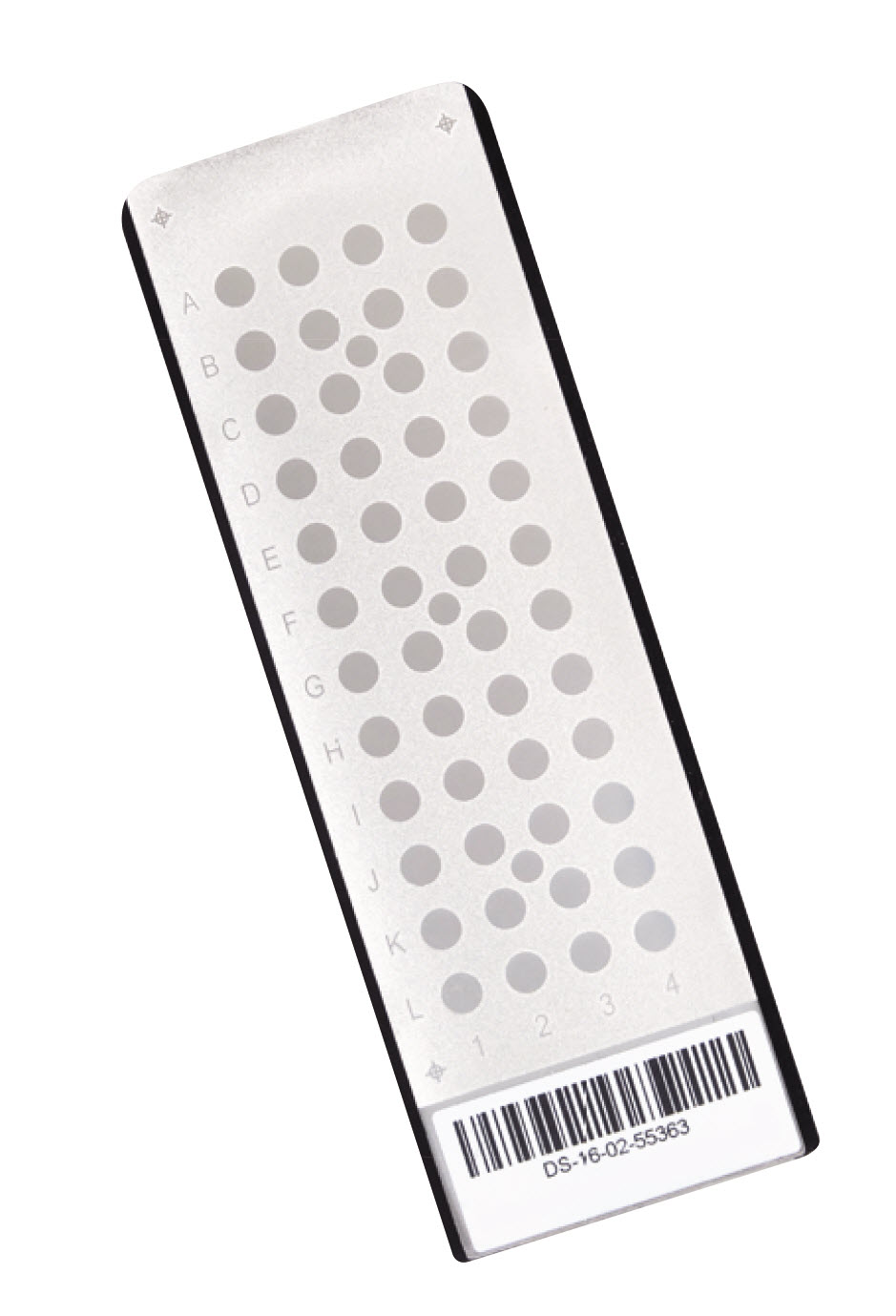
Disposable Target Slides
Each VITEK MS disposable target slide has a unique barcode to ensure clear traceability, flexibility, and greater confidence with no manual data entry. Divided into 3 separate acquisition groups with a calibration spot for each group, each slide can be used up to 3 times, once for each group, identifying up to 48 organisms per slide. Each box includes 32 MS-DS target slides.
- No cleaning requried: eliminates possible contamination that could lead to erroneous results or safety issues
- Each slide comes with three sections of 16 (48 samples) enabling the flexibility of small or large sample run sizes
- Up to 4 target slides (192 samples) can be run on the instrument at one time
VITEK PICKME™
VITEK PICKME is intended to pick and smear bacteria and yeast colonies on VITEK MS target slides.
Each VITEK PICKME Pen is reusable. VITEK PICKME Nib packs contain 18 trays with 96 nibs in each tray.
Ready-To-Use Reagents
The VITEK MS uses ready-to-use, light stable matrix solution (MS-CHCA) to save you time on reagent preparation. Formic Acid (MS-FA) is needed for yeast identification and is already allocated in small vials ready for use on VITEK MS disposable target slides.
- Optimize workflow and overall laboratory efficiency
- No extra reagent preparation needed
- MS-CHCA matrix and MS-FA are placed directly on the target slide
VITEK MS-CHCA matrix is added directly to the organism on the target slide. It absorbs energy from the VITEK MS laser and transfers it to the microorganisms to enable ionization. MS-CHCA is ready-to-use and conveniently packaged. Each box contains 5 vials of 0.5 mL VITEK MS-CHCA.
VITEK MS-FA is used to pretreat yeasts to facilitate identification. FA is added directly to yeast organisms on the target slide and is pre-allocated in ready-for-use vials. Each box contains 5 vials of 0.5 mL VITEK MS-FA.
Complete Reagent Kits
The VITEK MS Reagent kits contain reagents and disposables designed for use with the VITEK MS system.
Mycobacterium/Nocardia and Mold Reagent Kits
The VITEK MS Mycobacterium/Nocardia and Mold reagent kits contain all reagents necessary for simple inactivation and extraction for identification of these organisms on the VITEK MS. The protocols allow easy, quick and safe sample preparation.
VITEK MS MYCOBACTERIUM/NOCARDIA KIT IVD
The VITEK MS MYCOBACTERIUM/NOCARDIA KIT provides the reagents and consumables needed to process samples by protein extraction and inactivation for Mycobacterium and Nocardia identification. Each kit contains 100 tests in aliquots of 25.
To process samples for Mycobacterium from liquid media, use the additional consumables provided in the VITEK MS LIQUID MYCO SUPPLEMENTAL KIT. Each kit contains 100 tests.
The VITEK MS MOULD KIT provides the reagents and consumables needed to process samples by protein extraction and inactivation for mold identification from agar plates. The kit uses ethanol, formic acid and acetonitrile to inactivate molds and extract their proteins. Each kit contains 100 tests.
The VITEK MS Specimen rack is an autoclavable processing rack that ensures easy handling of Mycobacterium, Nocardia, and mold samples during sample inactivation and processing. This rack provides a secure plate to store tubes during extraction.
| Description | Ref. # | # of tests/kit | |
| Reagents | VITEK MS-DS Slides | 410893 | 1,536 |
| VITEK PICKME Pen | 423546 | N/A | |
| VITEK PICKME Nibs | 423551 | 1,728 | |
| VITEK MS CHCA | 411071 | 2,500 | |
| VITEK MS FA | 411072 | 5,000 | |
| VITEK MS Orange Gel | 411721 | N/A | |
| Mycobacteria Nocardia Mold Extraction Kits | VITEK MS MYCOBACTERIUM/NOCARDIA KIT IVD | 415659 | 100 |
| VITEK MS MOULD KIT IVD | 415680 | 100 | |
| VITEK MS LIQUID MYCO SUPPLEMENTAL KIT | 421564 | 100 | |
| Mycobacteria Nocardia Mold Ancillary Item | VITEK MS Specimen Rack | 421720 | N/A |
Technical Resources
Current list of FDA 510(k) Cleared Organisms
Statement of Inactivation of Molds by Sample Processing
Statement of Inactivation of Mycobacteria, Nocardia during Sample Processing
Posters
Flexible Clinical Workflow for Mycobacteria and Nocardia for VITEK® MS (MALDI-TOF): Reliable, Safe, and Efficient for Identification of Positive Patient Samples from Different Media Types
Reproducibility of the New bioMérieux VITEK® MS V3.0 Database for Identification of Moulds, Mycobacterium and Nocardia
Identification of a characterized challenge set of moulds, Mycobacterium and Nocardia strains using the new bioMerieux VITEK® MS V3.0 database
New innovative tool for easy colony picking and sample preparation for MALDI-TOF
Publications
VITEK MS IVD V3.0
Routine identification of Nocardia species by MALDI-TOF mass spectrometry. Girard V, Mailler S, Polsinelli S, et al.Diag Microbiol Infect Dis. 2017;87(1):7-10. doi:10.1016/j.diagmicrobio.2016.09.024
Identification of mycobacterium spp. and Nocardia spp. from solid and liquid cultures by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Girard V, Mailler S, Welker M, et al. Diag Microbiol Infect Dis. 2016;86(3):277-283. doi:10.1016/j.diagmicrobio.2016.07.027
Comparison of sample preparation methods, instrumentation platforms, and contemporary commercial databases for identification of clinically relevant mycobacteria by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Wilen CB, McMullen AR, Burnham C-AD. J Clin Microbiol. 53(7):2308–2315. doi:10.1128/JCM.00567-15
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry using the VITEK® MS system for rapid and accurate identification of dermatophytes on solid cultures. De Respinis S, Monnin V, Girard V, et al. J Clin Microbiol. 2014:52(12):4286-4292. doi:10.1128/JCM.02199-14
Rapid inactivation of mycobacterium and Nocardia species before identification using MALDI-TOF mass spectrometry. Dunne WM Jr, Doing K, Miller E, et al. J Clin Microbiol. 2014:52(10):3654-3659. doi:10.1128/JCM.01728-14
VITEK MS IVD V2.0 (In Vitro Diagnostic Applications)
Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) for rapid identification of micro-organisms in the routine clinical microbiology laboratory. Wattal C, Oberoi JK, Goel N, et al. Eur J Clin Microbiol Infect Dis. 2017:36(5):807-812. doi:10.1007/s10096-016-2864-9
Comparison of VITEK® MS and MALDI Biotyper® for identification of Actinomycetaceae of clinical importance.Ferrand J, Hochard H, Girard V, et al. J Clin Microbiol. 2016:54(3):782-784. doi:10.1128/JCM.02758-15
MALDI-TOF mass spectrometry for differentiation between Streptococcus pneumoniae and Streptococcus pseudopneumoniae. van Prehn J, van Veen S, Schelfaut J, Wessels E. Diagn Microbiol Infect Dis. 2016;85(1):9-11. doi:10.1016/j.diagmicrobio.2016.01.012
Using matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) complemented with selected 16S rRNA and gyrB genes sequencing to practically identify clinical important viridans group streptococci (VGS). Zhou M, Yang Q, Kudinha T, et al. Front Microbiol. 2016;7:1328. doi:10.3389/fmicb.2016.01328
Assessment of reproducibility of matrix-assisted laser desorption ionization–time of flight mass spectrometry for bacterial and yeast identification. Westblade LF, Garner OB, MacDonald K, et al. J Clin Microbiol. 2015;53(7):2349–2352. doi:10.1128/JCM.00187-15
Comparative evaluation of two matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) systems, VITEK® MS and Microflex LT, for the identification of Gram- positive cocci routinely isolated in clinical microbiology laboratories. Lee M, Chung HS, Moon HW, Lee SH, Lee K. J Microbiol Methods. 2015;113:13-15. doi:10.1016/j.mimet.2015.03.020
Performances and reliability of Bruker Microflex LT and VITEK MS MALDI-TOF mass spectrometry systems for the identification of clinical microorganisms. Bilecen K, Yaman G, Ciftci U, Laleli YR. Biomed Res Int. 2015;Article ID 516410:18 pages. doi:10.1155/2015/516410
Comparison and optimization of two MALDI-TOF MS platforms for the identification of medically relevant yeast species.Pence MA, McElvania TeKippe E, Wallace MA, Burnham CA. Eur J Clin Microbiol Infect Dis. 2014;33(10):1703-12. doi:10.1007/s10096-014-2115-xhttps://doi.org/10.1007/s10096-014-2115-x
MULTICENTER EVALUATIONS OF THE VITEK MS IVD V2.0 SYSTEM
Multicenter validation of the VITEK® MS v2.0 MALDI-TOF mass spectrometry system for the identification of fastidious gram-negative bacteria. Branda JA, Rychert J, Burnham CA, et al. Diag Microbiol Infect Dis. 2014;78(2):129-31. doi:10.1016/j.diagmicrobio.2013.08.013
Multicenter evaluation of the VITEK® MS system for mass spectrometric identification of non-Enterobacteriaceae Gram-negative bacilli.Manji R, Bythrow M, Branda J, et al. Eur J Clin Microbiol Infect Dis. 2014;33(3):337-346. doi:10.1007/s10096-013-1961-2
Multi-centre evaluation of mass spectrometric identification of anaerobic bacteria using the VITEK® MS system. Garner O, Mochon A, Branda J, et al. Clin Microbiol Infect. 2014;20(4):335-339. doi:10.1111/1469-0691.12317
Multicenter evaluation of the VITEK MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of Gram-positive aerobic bacteria. Rychert J, Burnham CA, Bythrow M, et al. J Clin Microbiol. 2013:51(7):2225-2231. doi:10.1128/JCM.00682-13
Multicenter study evaluating the VITEK MS system for identification of medically important yeasts. Westblade LF, Jennemann R, Branda JA, et al. J Clin Microbiol. 2013;51(7):2267-2272. doi:10.1128/JCM.00680-13
Identification of Enterobacteriaceae by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using the VITEK® MS system. Richter SS, Sercia L, Branda JA, et al. Eur J Clin Microbiol Infect Dis. 2013;32(12):1571-1578. doi:10.1007/s10096-013-1912-y