VIDAS® C. difficile GDH and TOXIN A & B
Automated and cost-efficient C. difficile testing in one platform
The VIDAS® C. difficile GDH and TOXIN A & B (CDAB) are two complementary tests used as an aid in the diagnosis of C. difficile associated disease.
- Automated and easy to use for rapid results
- Flexible solution adapted to small or large volumes
- Improve diagnosis with an IDSA & SHEA guidelines recommended process
>> Download our C. Diff Clinical Booklet: From Diagnosis to Outbreak Mangement
Situation
C. difficile "urgent" threat today
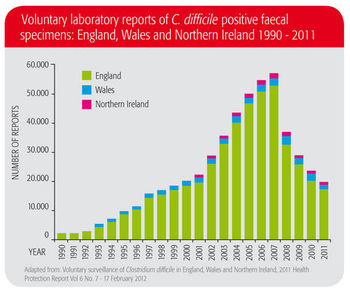
Once upon a time, Clostridium difficile was considered little more than a clinical nuisance. Today, C. difficile is one of our most challenging healthcare-associated infections (HAI), with nearly half a million cases of C. difficile infections (CDI) per year in the United States.1 In most cases, C. difficile is contracted in the healthcare setting and is connected to antibiotic treatment. C. difficile infection incurs costly treatment, patient isolation and longer hospital stays. In addition to the impact on patients themselves, this means high economic burden on hospitals and healthcare systems. C. difficile is at historically high levels worldwide, according to the Centers for Disease Control. Hyper-virulent strains are emerging and we are seeing severe outbreaks, and morbidity and mortality rising at alarming rates.3
There is also evidence that C. difficile transmission is spreading out of the healthcare setting into the community.4 In fact, the CDC classified C. difficile in 2013 as an “urgent threat”, meaning “an immediate public health threat that requires urgent and aggressive action”.3
Challenge
Managing C. difficile from prevention to epidemiological trending
Fighting today’s complex healthcare challenges like C. difficile requires a multi-faceted approach. Healthcare institutions, medical professionals, microbiologists, patients – we are all involved. At bioMérieux, we are your dedicated partners, bringing our longstanding, recognized expertise in microbiology, and our innovative laboratory solutions to support your actions.
C. difficile infection management starts with prevention and moves along a continuum including diagnosis, treatment/surveillance, outbreak management and epidemiological trending. The microbiology lab is challenged to provide fast, accurate diagnostic and surveillance answers as well as reliable records for trending. There are a number of needs to ensure better responses to the challenges at every stage of C. difficile infection management:
Prevention/infenction control needs:
- Effective hygiene and cleansing procedures
- Antimicrobial stewardship: Adapted therapies to be implemented at the right time
- Education and involvement of healthcare staff (e.g., correct hygiene/hand-washing, antibiotic use, etc.)
- Effective, environmental control tests
Diagnosis needs:
- Rapid, accurate, easy and cost-effective diagnostic tools including instruments and reagents
- Good testing algorithm guidelines
- Increased awareness
Outbreak management needs:
- Fast and easy genotyping tools for C. difficile strain genotyping and outbreak management
- Reliable data management to optimize information flow
Epidemiology needs:
- Accurate, complete and easy-to-access data collection and reporting to track trends
- Good information flow across regions
bioMerieux's Commitment
Our dedication to fighting C. difficile
|
bioMérieux knows labs With more than 50 years’ experience working with microbiology labs, we’ve been at your side throughout the evolution of challenges facing labs around the world – including the emergence of the C. difficile threat. |
Advances are needed at all levels (medical, scientific, public policy, etc.) to ensure continual improvement in the management of C. difficile infections. In addition to our comprehensive approach to C. difficile diagnostics, bioMérieux contributes through scientific and educational initiatives like these:
- The World Forum on Healthcare Associated Infection and Antimicrobial Resistance, organized by bioMérieux, brings together over 70 international leaders in the field to discuss and drive new strategies to respond to HAI/Resistance challenges.
- Our activities for Antibiotic Awareness Week and Clostridium difficile Awareness Month.
- Our informational publications include Clostridium difficile Infections from diagnosis to Outbreak Management and ANTIMICROBIAL STEWARDSHIP: A Practical Guide to Implementation in Hospitals.
- We develop educational partnerships with leading associations like the French National Observatory for Epidemiology of Bacterial Resistance to Antimicrobials (ONERBA).
- We support young microbiologists through grants and awards – knowing that tomorrow’s leaders in the field will help us improve responses to our greatest healthcare challenges.
References
1. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34.
2. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55(Suppl 2):S88–92. doi:10.1056/NEJMoa1408913.
3. Centers for Disease Control and Prevention: ANTIBIOTIC RESISTANCE THREATS in the United States, 2013. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Updated April 10, 2017. Accessed March 23, 2018.
4. Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. A case–control study of community-associated Clostridium difficile infection. J Antimicrobial Chem. 2008;62(2):388-396. doi:10.1093/jac/dkn163
Automated C. difficile Tests
The VIDAS C. difficile GDH assay is an enzyme-linked fluorescent assay technique (ELFA) qualitative test that detects the C. difficile antigen, glutamate dehydrogenase (GDH), as a screen for the presence of C. difficile in stool specimens from persons suspected of having C. difficile infection (CDI).
The VIDAS C. difficile TOXIN A & B (CDAB) is an enzyme-linked fluorescent assay technique (ELFA) qualitative test that detects the C. difficile toxin A and toxin B in stool specimens from persons suspected of having C. difficile infection (CDI).
The VIDAS® C. difficile solution will rapidly provide the information needed to help you identify and contain the spread of the infection.¹ This will help decrease patient morbidity and mortality rates, and reduce the economic and human burden of C. difficile on hospitals and healthcare systems.²
“VIDAS® Clostridium difficile GDH is an automated test, allowing easier interpretation and traceability of results.”
-Comparison of the VIDAS® C. difficile GDH and the GDH component of the C. diff Quik Chek Complete for detection of Clostridium difficile in stools. C. Eckert, et. al. (Paris, FR)ECSMID: 29.04.2013
Solution Benefits
The ONLY FDA cleared automated C. difficile GDH and TOXIN A & B assays
- Eliminate subjective test result interpretation
- Easy workflow for either individual or batch testing
- Load and go testing with minimal hands-on time
- Automatically store and send results to LIS
Quickly rule out negative patients
- Screen samples with a high sensitivity and high NPV GDH test3
- Get GDH and CDAB test results < 1hr
Avoid overdiagnosis of C. difficile infections4
- Use a high PPV toxin test as an aid for diagnosing C. difficile associate disease4,5
- Detect both toxigenic C. difficile producing toxin A and toxin B with a single toxin test
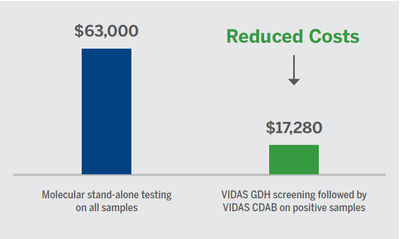
Cost effective3,6
- Potential annual test cost savings by using the two-step process with VIDAS C. difficile:
- Based on theoretical data/5 patients a day
- Assumes 80% rule-out with GDH
- Average price molecular assay $35/test
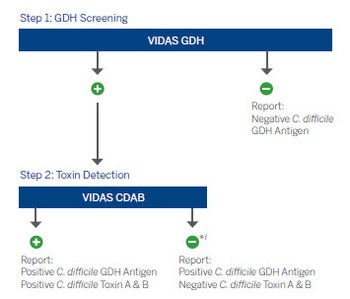
Algorithms
Improve C. difficile diagnosis with an IDSA & SHEA guidelines recommended process.3
*Positive GDH or molecular results, combined with negative toxin (CDAB) result may indicate possible colonization. Clinical interpretation of test results should be made taking into consideration the patient history and the results of any other tests performed.3-5
†Positive GDH result, combined with negative toxin (CDAB) result may be arbitrated by molecular testing (NAAT).3
bioMérieux's Complete C. difficile Solution

References
1. Fenner L, Widmer AF, Goy G, Rudin S, Frei R. Rapid and Reliable Diagnostic Algorithm for Detection of Clostridium difficile. J Clin Microbiol. 2008;46(1):328-330. doi:10.1128/JCM.01503-07
2. Siegel JD, Rhinehart E, Jackson M, Chiarello L, and the Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Am J Infect Control. 2007;35(10):S65-S164. doi:10.1016/j.ajic.2007.10.007
3. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018.
4. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175(11):1792-1801. doi:10.1001/jamainternmed.2015.4114
5. VIDAS® C. difficile GDH and VIDAS® C. difficile TOXIN A & B package inserts.
6. Ticehurst JR, Aird DZ, Dam LM, Borek AP, Hargrove JT, Carroll KC. Effective detection of toxigenic Clostridium difficile by a two-step algorithm including tests for antigen and cytotoxin. J Clin Microbiol. 2006;44(3):1145-1149. doi:10.1128/JCM.44.3.1145-1149.2006
| VIDAS® C. difficile GDH |
VIDAS® C. difficile TOXIN A & B |
|
| Reference | 30125-01 | 30118-01 |
| Tests/kit | 60 | 60 |
| Time to result | Within approximately 50 minutes | Within approximately 75 minutes |
| Sample type | Fecal specimen | Fecal specimen |
| Stool sample volume | 200 µL | 200 µL |
| Sample volume after pre-treatment | 300 µL | 300 µL |
| Calibration frequency | 28 days | 14 days |
| CPT Code | 87449 | 87324 |
|
VIDAS C. difficile Clinical Performance |
||||
|
|
Sensitivity |
Specificity |
Negative Predicitve Value (NPV) |
Positive Predictive Value (PPV) |
|
VIDAS C. difficile GDHa |
95.6% |
90.1% |
99.1% |
— |
|
VIDAS C. difficile TOXIN A & Bb |
88.3% |
99.8% |
98.4% |
98.1% |
aCompared to Culture
bCompared to Cellular Cytotoxicity
See package insert for more details.
To have access to more resources and strengthen your expertise, visit the tech library at www.mybiomerieux.com.
Medical Education Booklet
CLOSTRIDIOIDES DIFFICILE INFECTIONS (CDI)
From Diagnosis to Outbreak Management
This booklet concentrates on the key information needed to understand, diagnose, control and treat CDI, taking into account the large expansion of knowledge that has occurred in response to the emergence of C. difficile in the first decade of this millennium as a global threat.
Related Publications
ECCMID 2014
J. Van Broeck, E. Ngyuvula Mantu, K. Soumillion, M. Delmée . Evaluation of the impact of freezing on positive Clostridium difficile stools using VIDAS® GDH & VIDAS® CD toxins A/B, Liaison GDH, Liaison toxins A&B and C. DIF QUIK CHEK Complete®.
ECCMID 2013 Abstract R2753
L. De Cooman, S. Drieghe, I. Leroux-Roels, G.Glaeys. J. Boelens. Evaluation of the VIDAS® Gultamase Dehydrogenase Test (bioMérieux) for algorithm-based Clostridium difficile testing.
ECCMID 2013 Poster P187
C.Eckert, O. Said, C. Rambaud, N. Poccardi, B. Burghoffer, V. Lalande, F. Barbut. Comparison of the VIDAS® C. difficile GDH and the GDH component of the C. diff Quik Chek Complete for detection of Clostridium difficile in stools.
ECCMID 2013 Poster 1872
D. Achleiter, A. Wutscher, M. Hell. Evaluation of the new VIDAS GDH (ELFA) Test and VIDAS Clostridium difficile Toxin A&B Test compared to a 3-Step Toxin B-PCR based-algorithm at a University Hospital.
ECCMID 2013 P2266
Davies, K. A., Bosomworth, C. E., Carricajo, A., Adam, T. and Wilcox, M.H. Comparison of VIDAS® GDH automated immunoassay with Cepheid GeneXpert® C. difficile PCR assay and an in-house PCR assay for GluD, for the detection of C. difficile in faecal samples.
Guidelines
The Updated 2017 C. difficile diagnosis guidelines recommend the use of a multistep protocol, including IA testing, regardless of the preagreed institutional criteria for patient stool sample submission.
| Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) | 2017 | Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Soceity for Healthcare Epidemiology of America (SHEA). http://www.idsociety.org/Guidelines/Patient_Care/IDSA_Practice_ Guidelines/Infections_By_Organ_System-81567/Gastrointestinal/Clostridium_difficle/ |
| Association for Professionals in Infection Control and Epidemiology (APIC) | 2013 | Guide to Preventing Clostridium difficile Infections. http://apic.org/Professional-Practice/Implementation-guides |
| Center for Disease Control and Prevention (CDC) | 2018 | Surveillance Reporting for Enrolled Facilities. https://www.cdc.gov/nhsn/enrolled-facilities/index.html |
PRN: 18-0203-00